Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is an effective treatment for acute lymphoblastic leukemia (ALL) resulting in long-term remission.
Despite advances in therapy, disease progression remains the major cause of mortality following HSCT accounting for 20-50% of all deaths. The central nervous system (CNS) is the most common extra-medullary site of disease progression after transplant in ALL.
The established practice of CNS prophylaxis for ALL has largely been modeled in the pediatric population, given the higher incidence of ALL in this age group compared with adults.
Although the use of CNS prophylaxis as part of the upfront treatment for ALL has led to significant decreases in CNS relapse and improved outcomes overall, the routine use of post-HSCT prophylactic CNS therapy as a strategy to prevent CNS relapse after transplant in adult patients is still controversial. Studies that utilized post-HSCT CNS prophylaxis have reported disparate results and there is no generalized consensus regarding the use of post-transplant CNS prophylaxis to prevent relapse.
Methods
In order to assess the current practice of post-HSCT prophylactic CNS therapy in preventing CNS relapse in ALL patients, we carried out a survey among EBMT centres to describe intrathecal (i.t.) prophylaxis or irradiation in HSCT for ALL in both patients with or without CNS manifestations at any time of disease (first or second complete remission [CR1, CR2] and relapsed/refractory [R/R] ALL).
EBMT centres were asked whether they usually give i.t. prophylaxis or cranial irradiation before and after HSCT, and which drugs they use.
Results
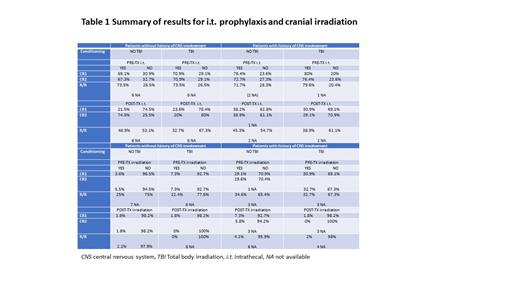
Of 410 invited centres, 55 (13%) replied to the survey. Tables 1 shows the number of centres which use i.t. prophylaxis or cranial irradiation. Most of the 55 centres used both pre-and post i.t. prophylaxis and triple prophylaxis (steroids, cytarabine [AraC] and methotrexate [MTX]) was the most frequently adopted.
As expected in the pre-transplant phase, almost 70% of centres use i.t. chemotherapy irrespective of the phase of the disease or of the use of total body irradiation (TBI) as conditioning regimen. In the post-transplant phase, the rate of i.t prophylaxis varies according to disease phase, TBI use, and history of CNS involvement pre HSCT.
In patients w/o history of CNS involvement in CR1, i.t. prophylaxis is given in 23.6% and 21.5% (with or w/o TBI use, respectively). Notably in CR2 pts, i.t. prophylaxis is given in 20% of cases if the conditioning regimen includes TBI, and in 74.5% of cases if TBI is not used; 32.7% and 46.9% R/R pts (with or without TBI use, respectively) are given i.t. prophylaxis.
In the setting of patients with history of CNS involvement in CR1, i.t. prophylaxis is given in and 30.9% and 38.2% (with or without TBI use, respectively); in CR2 pts, i.t. prophylaxis is given in 38.9% of cases if the conditioning regimen does not include TBI, and in 29.1% of cases if TBI is used; 38.9% and 45.3% R/R pts (with or without TBI use, respectively) are given i.t. prophylaxis.
Pre-transplant cranial irradiation is planned in less than 10% of centres in CR1 and CR2 patients without CNS involvement, irrespective of the use of TBI in the conditioning regimen, while it is used in 22.4% and 25% of patients with R/R disease with or without TBI use for transplant, respectively. In the setting of patients with CNS involvement, among those in CR1 and CR2, approximately 30% receive pre-transplant cranial irradiation irrespective of TBI use, while 32.7% and 34.6% of patients receive cranial irradiation (with or without TBI use, respectively). Its use after transplant is uncommon irrespective of disease status at treatment.
Conclusions
CR2 pts (without CNS involvement) seem to be at higher risk of CNS relapse if TBI is not included in the conditioning regimen.
R/R pts are always considered at higher risk for CNS relapse if TBI is not included in the conditioning regimen.
Cranial irradiation before treatment is used in almost 1 out of 3 pts with CNS involvement despite the use of TBI as the conditioning regimen, and 1 out 4 R/R pts without CNS involvement at transplant.
The rate of cranial irradiation after transplant is extremely low, irrespective of diseases status, previous CNS involvement or TBI use.
In conclusion, the use of i.t. prophylaxis after HSCT in patients with or without CNS involvement is variable among European transplant centres, also in the era of novel therapies for patients with ALL.
Disclosures
Bug:Novartis: Honoraria; Jazz: Honoraria, Other: Travel Grant; BMS: Honoraria; Gilead: Honoraria, Other: Travel Grant; Pfizer: Honoraria; Neovii: Other: Travel Grant. Esteve:Pfizer: Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Abbvie: Consultancy; Gilead: Consultancy; Kronos Bio: Research Funding; Astellas: Consultancy. Savani:Takeda Development Center Americas, Inc. (TDCA): Current Employment. Versluis:AbbVie: Honoraria; ExCellThera: Consultancy. Mohty:JAZZ PHARMACEUTICALS: Honoraria, Research Funding. Ciceri:ExCellThera: Other: Scientific Advisory Board .

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal